RESEARCH ARTICLE
Chronic Obstructive Pulmonary Disease (COPD) and Asthma-Chronic Obstructive Pulmonary Disease Overlap Syndrome (ACOS) are Risk Factors for Cryptococcosis
Aline B. Mahler Pereira1, Alexandre P. Rogerio1, *
Article Information
Identifiers and Pagination:
Year: 2020Volume: 11
First Page: 1
Last Page: 4
Publisher Id: TOALLJ-11-1
DOI: 10.2174/1874838402011010001
Article History:
Received Date: 08/10/2019Revision Received Date: 26/03/2020
Acceptance Date: 12/04/2020
Electronic publication date: 04/06/2020
Collection year: 2020
open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: https://creativecommons.org/licenses/by/4.0/legalcode. This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Cryptococcosis is a fungal infection of global importance affecting the central nervous system and other organs such as the lungs. The severity of cryptococcosis is largely dependent on the integrity of the host immune system. The protection to cryptococcosis is associated with Th1 immune response while Th2 results in susceptibility to Cryptococcus infection. Asthma is a chronic inflammatory disease commonly coordinated by Th2 immune response. The airway inflammation in Chronic Obstructive Pulmonary Disease (COPD) patients is characterized by increased neutrophils, macrophages, proteases, IL-6, IL-8, and Th1 cytokines. Asthma-Chronic Obstructive Pulmonary Disease Overlap Syndrome (ACOS) patients present phenotype that shares Th1 (COPD) and Th2 (asthma). There are several risk factors associated with Cryptococcus infection, including smoking, that cause airway remodeling and dysregulated and damaging airway inflammation.
1. CRYPTOCOCCOSIS IN THE LOWER RESPIRATORY SYSTEM
Cryptococcosis is an infection of global importance with cryptococcal meningoencephalitis being the most deadly symptom mainly in patients with Human Immunodeficiency Viruses (HIV) [1]. The genus Cryptococcus comprises over 30 yeast- like fungal species. However, the majority of human infections are caused by C. neoformans and C. gattii [2]. C. neoformans is commonly present in avian droppings while C. gattii is found mainly in decayed woods inside trunk hollows of diverse tree species. Both C. neoformans and C. gattii infections start with inhalation of the fungus in the airways. From the lungs, the fungus can spread to other organs of the body especially to the central nervous system [3]. C. neoformans and C. gattii have several virulence factors, especially the capsule that consists of high molecular weight polysaccharides, mainly glucuronoxylomannan (GXM) and, to a lesser extent, galactoxylomannan (GalXM) and mannoproteins (MP). Other virulence factors include melanin production, which protects the C. neoformans and C. gattii against phagocytosis, and the production of various enzymes such as proteases, ureases, superoxide dismutase and phospholipase B, which facilitates fungal invasion of tissues [4-7]. The severity of cryptococcosis is largely dependent on the integrity of the host immune system and the characteristics of each variety of C. neoformans and C. gattii [8].
2. ASTHMA, COPD AND ACOS
Asthma is a chronic inflammatory disease common worldwide, which is coordinated by Th2 immune response. Of the numerous smoking-related disorders, the most common is chronic obstructive pulmonary disease (COPD) and the most deadly is lung cancer [9]. COPD is characterized by chronic airway obstruction leading to a progressive and irreversible decline in lung function as a consequence of activation of airway inflammation with release of inflammatory mediators (IL-6, IL-8, and Th1 cytokines) and proteolytic enzymes (metalloproteinases), and continuous cells recruitment (neutrophils, macrophages and others). Of note, COPD patients can also present a Th2 phenotype with an increase of eosinophils in sputum and blood. The allergic phenotype in COPD patients has been associated with an increased risk of airways exacerbations [10, 11]. In smokers with asthma, the sputum airway inflammation is often reported to be eosinophilic, neutrophilic or mixed granulocytic [12-14]. However, it is possible to have no association between smoking and inflammatory phenotypes in asthma [15]. Asthma-chronic obstructive pulmonary disease overlap syndrome (ACOS) patients present a mixture of Th1 immune response (characteristic of COPD) and Th2 (characteristic of asthma) immune response [16, 17].
3. SMOKING AND CRYPTOCOCCOSIS
C. neoformans can cause infection in individuals with normal but preferentially immunocompromised hosts such as HIV-infected patients [18]. On the other hand, C. gattii infection occurs mainly in healthy individuals [19-23]. HIV-infected patients with the cryptococcal infection have shown increase of IL-6, IFN-γ, TNF-α and IL-8 production in the cerebrospinal fluid from survivors compared to nonsurvivors [24, 25]. The protection to cryptococcosis is associated with Th1 immune response while Th2 results in susceptibility to infection [10, 11]. Studies on asthmatics demonstrated controversial results, while the IgA reactivity to C. neoformans in the Bronchoalveolar Lavage Fluid (BALF) was found to be greater in asthmatic children compared with non-asthmatic children [26]. In non-asthmatic and asthmatic adults, no significant alteration was observed in IgG seroreactivity against C. neoformans [27]. More studies are needed to demonstrate the susceptibility to cryptococcosis asthma patients. There are several factors associated with C. neoformans and C. gattii infections such as rheumatic disorders, malignancy, diabetes mellitus, cirrhosis, hepatitis B virus infection as well as smoking [1, 28, 29]. HIV-infected patients who were current smokers demonstrated an increased risk for C. neoformans infection [30, 31]. In another study, MacDougall et al. [32] observed an increase of C. gattii infection in smokers with an immunosuppressive state, induced by oral corticosteroid use or with invasive cancers. Nicotine, a component identified in cigarette tobacco smoke, demonstrated immunosuppressive effects and could be associated with an increase of susceptibility to cryptococcal infections [22]. Additionally, tobacco contains a large number of chemicals and the consequent absorption of these compounds directly into the lungs or bloodstream, during smoking, could serve as precursors for the synthesis of several substances including melanin [28, 33]. Melanization of C. neoformans is associated with more virulent, increased resistance to host defenses [34] and reduced susceptibility to antifungal agents [35]. Taken together, the increased susceptibility to C. neoformans and C. gattii infections induced by smoking could be associated to its effects on the respiratory system (impairments of the protective barriers, inhibition of mucociliary clearance, airway remodeling, excessive and continuous airway inflammation and destruction of the lung parenchyma) [36, 37]. Besides, smoking can provide substances with immunosuppressive effects or with the potential to melanize fungus cells, thus increasing the virulence and burden of fungus in the airways and consequently in other tissues such as the central nervous system (Fig. 1).
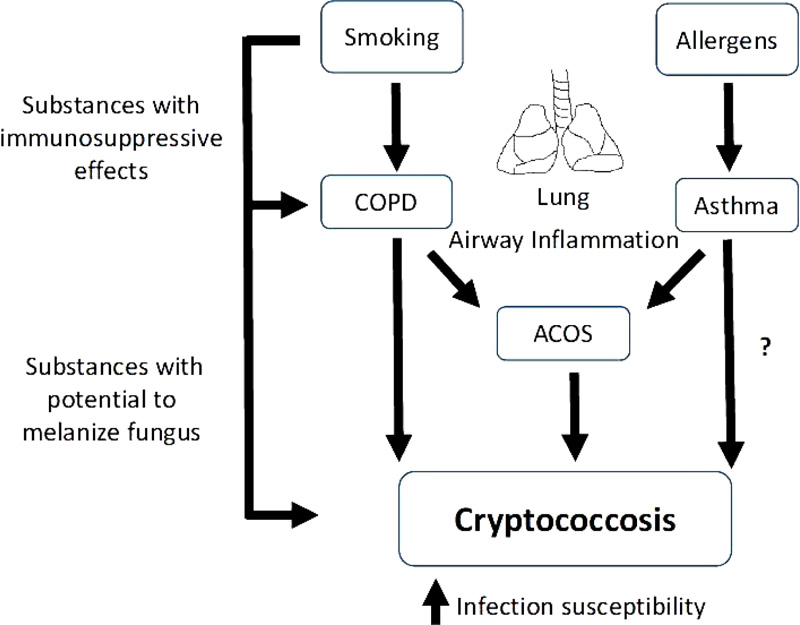 |
Fig. (1). Smoking can increase susceptibility to C. neoformans and C. gattii infections in COPD and ACOS patients as a consequence of its effect on the airway inflammation and the fungus. |
CONCLUSION
Smoking weakens pulmonary function and provides substances associated with fungal resistance mechanisms that can contribute towards the enhanced risk of cryptococcosis in smokers or ex-smokers mainly those individuals with COPD and ACOS.
AUTHORS' CONTRIBUTIONS
All authors contributed to critiquing the manuscript. All authors have read and approved the final manuscript.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.




